Published online Dec 28, 2013. doi: 10.5316/wjn.v3.i4.138
Revised: August 19, 2013
Accepted: September 14, 2013
Published online: December 28, 2013
Processing time: 202 Days and 1.9 Hours
Following injury, the axons of the mammalian central nervous system do not regenerate. Many studies have aimed at understanding the mechanisms that prevent axonal regeneration and at designing ways to overcome the obstacles preventing axonal regrowth. These studies have identified numerous proteins as promoters of axonal regeneration. In this minireviews, we focus on neuritin as a therapeutic candidate for promoting axonal regeneration. Neuritin was first identified as a neuronal-activity-inducible gene product in the rat brain. The overexpression of neuritin in neurons or the application of neuritin to neurons induces neuritogenesis, neurite arborization, and axonal elongation both in vitro and in vivo. These morphological changes are often observed during the first step of axonal regeneration. Indeed, neuritin expression increases during axonal regeneration in the peripheral nervous system (PNS). Conversely, in a mouse model of diabetes mellitus, neuritin expression decreases in the PNS, and this reduced expression may result in deficient axonal regeneration. Neuritin is induced in the hippocampal dentate gyrus after temporal lobe epilepsy or brain ischemia; however, in these conditions, neuritin induction may exacerbate brain dysfunction through mossy fiber sprouting. Together, these findings support the hypothesis that tightly controlled regulation of neuritin may be required for the treatment of each unique axonal pathology.
Core tip: Neuritin has been shown to be an activity-regulated protein in neurons. Its expression also increases after neuronal damage. Neuritin induces neuritogenesis, arborization, and axonal elongation. These functions may be beneficial for axonal regeneration after nerve injury. Here, we review neuritin as a therapeutic candidate for promoting axonal regeneration in the peripheral and central nervous systems. We also discuss the possible involvement of neuritin in mossy fiber sprouting after epileptic seizures or brain ischemia.
- Citation: Shimada T, Sugiura H, Yamagata K. Neuritin: A therapeutic candidate for promoting axonal regeneration. World J Neurol 2013; 3(4): 138-143
- URL: https://www.wjgnet.com/2218-6212/full/v3/i4/138.htm
- DOI: https://dx.doi.org/10.5316/wjn.v3.i4.138
After neuronal injury, axonal regeneration is not observed in the mammalian central nervous system (CNS). The regeneration of an axon itself is the only way to recover neuronal function after CNS injury. For decades, many investigators have tried to develop methods that promote axonal regeneration.
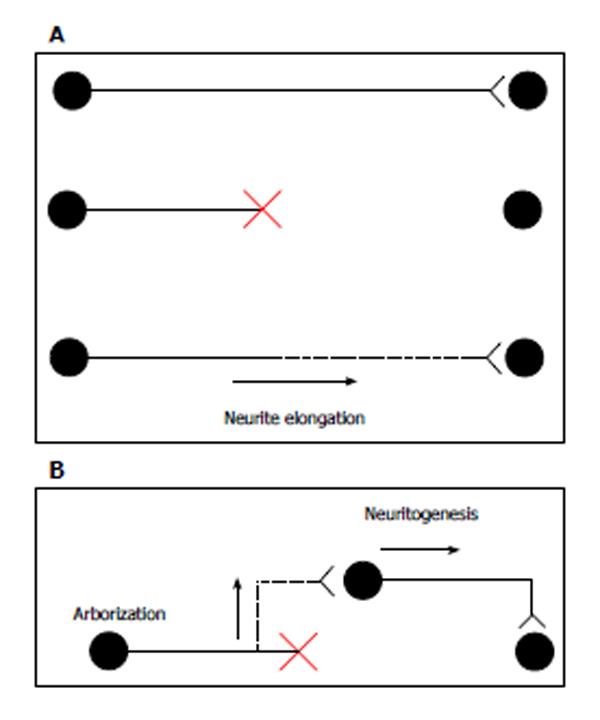
In canonical axonal regeneration, new growth is expected to occur from the tip of a transected axon and reinnervate its normal target. Because CNS neurons maintain the intrinsic ability of axonal outgrowth[1], the promotion of neurite outgrowth can contribute to the regrowth of axons (Figure 1A). In addition to canonical regeneration, other processes can achieve the functional recovery of transected neurons. New axon branches could arise from the axonal region close to the injury site, and these branches could connect with other newborn neurons near the lesion. Alternatively, neural precursor cell grafts enable the production of newborn neurons. New neurons close to the injury site are expected to extend their axons to the target organs. In this model, enhancements of neuritogenesis and arborization help form new axons and branches, respectively (Figure 1B). A few transplant models have succeeded in promoting axonal functional recovery[2,3]. Thus, axonal elongation, branch formation, and neuritogenesis may be crucial for axonal regeneration.
In this minireviews, we focus on the protein neuritin, which is one candidate for the promotion of axonal regeneration. The expression level of neuritin is altered in CNS and peripheral nervous system (PNS) diseases. In addition, the overexpression or application of neuritin promotes neurite formation, branch formation, and neurite elongation in cultured neurons. These changes may promote axonal regeneration.
Neuritin, which is also known as candidate plasticity gene 15 (CPG15), was first identified during a screen for neural-activity-regulated genes in the hippocampus[4]. Neuritin mRNA is predominantly expressed in the brain. Minor expression is observed in the lungs and liver[5]. Within the brain, the highest levels of expression are in the dentate gyrus of the hippocampus[5]. Expression of neuritin is increased by neural stimulation. Neuritin mRNA was increased both in the cerebral cortex by light[6], sensory experience[7], and exercise[8] and in the hippocampus by exercise[8] and electroconvulsive seizure[9]. Immunohistochemical analysis showed the expression of neuritin in the sciatic nerve[10] and facial motor neuron[11], indicating that neuritin is also expressed in the PNS.
An open reading frame of the neuritin sequence predicts a protein with 143 amino acids and a 27-amino-acid signal peptide. The 27 C-terminal amino acids contain a consensus cleavage signal that is found in glycosylphosphatidylinositol-anchored proteins. The predicted mature protein is a membrane-bound 12-kDa protein with no apparent phosphorylation or glycosylation site. Neuritin shows no significant homology with any known protein[5]. A portion of neuritin may be cleaved from the membrane and exists as a soluble form[12].
Neuritin protein is concentrated in the neuronal soma and is unevenly dispersed along neuritic projections in the rat brain[5]. Neuritin is expressed in puncta throughout cell bodies, dendrites, and axons, although its expression in axon terminals is particularly strong[13].
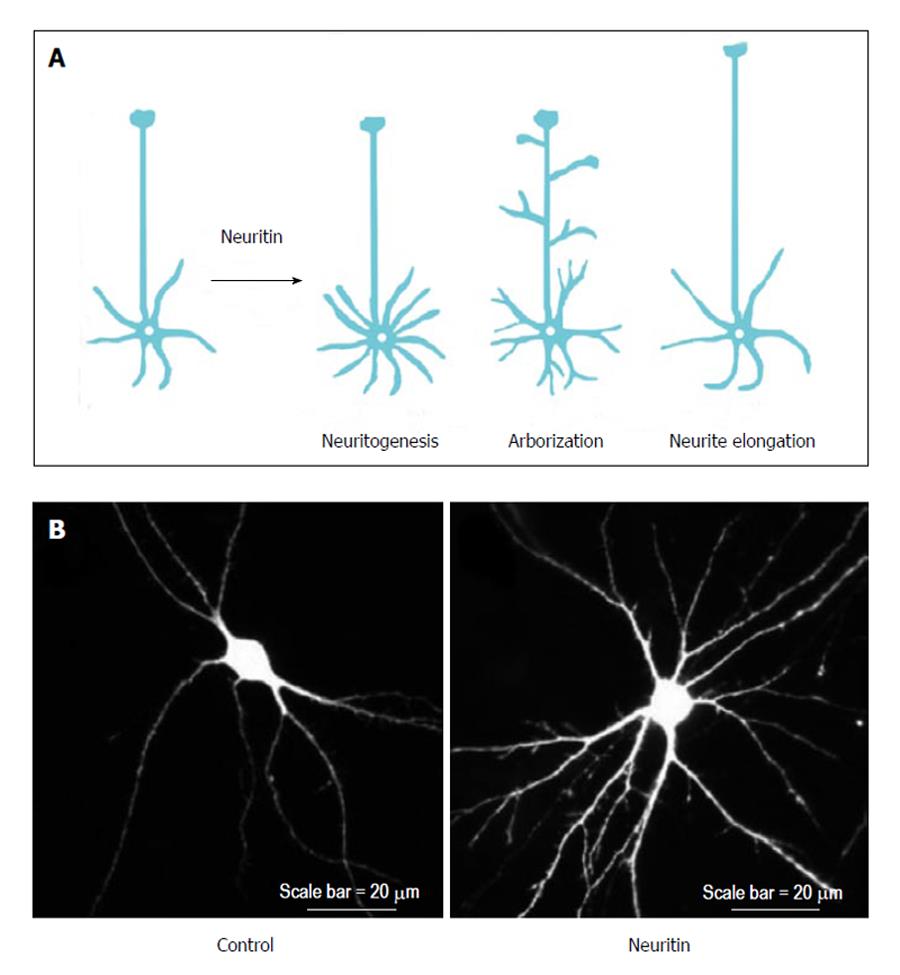
The neuronal functions of neuritin have been investigated primarily through studies involving the overexpression, gene silencing, and application of neuritin protein. These functions of neuritin can be categorized into the following three groups: neuritogenesis, neurite arborization, and neurite extension (Figure 2A).
After the administration of purified neuritin to cultured hippocampal and cortical neurons, neurons showed extensive dose-dependent neuritogenesis compared with control neurons[5,13]. In contrast, gene silencing of endogenous neuritin caused a robust reduction in nerve growth factor (NGF)-induced neurite formation[14]. Because NGF induces neuritin expression in dorsal root ganglion (DRG) neurons[10], NGF may promote neurite formation through neuritin expression; however, it remains unclear how NGF regulates neuritin expression. Furthermore, the application of a neutralizing antibody against neuritin to cultured cortical neurons significantly decreased the number of their primary dendrites and their total dendritic length[13]. These findings suggest that neuritin enhances neuritogenesis in developing neurons.
Neuritin can promote branch formation in neuronal dendrites. The overexpression of neuritin increased dendritic arbors in the optic tectal neurons of Xenopus tadpoles[15]. Cultured rat hippocampal neurons overexpressing neuritin also exhibited significant increases in dendrite arborization (Figure 2B). Furthermore, neuritin targets axons to promote branch formation. The overexpression of neuritin changed the dynamic behavior of axonal branches. In the neuritin-expressing Xenopus motoneurons, the axonal branches grew significantly faster than those of control neurons owing to increased branch formation and decreased branch retraction[16].
In addition to neuritogenesis and branch formation, neuritin promotes the elongation of axons and dendrites. Dissociated cortical neurons plated on neuritin-coated dishes extended longer neurites than those plated on bovine serum albumin-coated dishes[5]. In addition, dissected hippocampal explants co-cultured with neuritin-expressing cells showed significantly longer neurites than those co-cultured with control cells[17]. Conversely, down regulation of neuritin expression completely abolished NGF-induced neurite outgrowth. The longest neurite of neuritin-reduced neurons treated with NGF was comparable to that of control neurons cultured without NGF[10]. Thus, there has been progress in our understanding of neuritin’s functions, but by contrast, little is known about its mechanisms of action. A recent study has shed light on its signaling pathway (e.g., the effects of neuritin on cerebellar granule neurons is attenuated by ERK, Akt, or mTOR inhibitors[18]); however, further work will be needed to dissect the mechanistic contributions of neuritin to different forms of neuronal function.
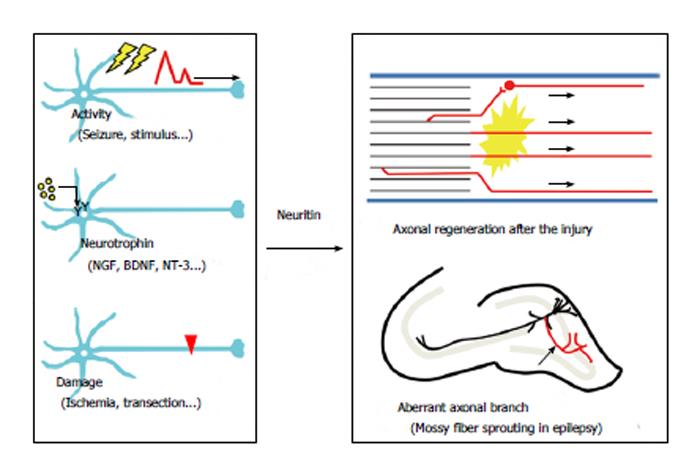
Neuritogenesis, axonal elongation, and axonal branching may be necessary for axonal regeneration. Accordingly, neuritin could contribute to axonal recovery. Several lines of evidence indicate that neuritin is involved in axonal regeneration in the PNS (Figure 3). In diabetes mellitus, axonal regeneration is nearly nonexistent in both experimental animal models[19,20] and clinical cases[21,22]. Streptozotocin (STZ) treatment causes experimental diabetic neuropathy in rats. Neuritin mRNA and protein have been shown to be significantly reduced in the DRG after STZ treatment[10]. The sections proximal to a ligature in the sciatic nerve exhibited a significant reduction in neuritin levels in diabetic rats compared with those in control rats, while distal sites did not[10]. These results suggest that the axonal transportation of neuritin to the ligature site may be reduced in diabetic rats, raising the possibility that experimental diabetic neuropathy could result from a deficiency in axonal regeneration caused by a decrease in neuritin expression.
What mechanism is involved in the reduction of neuritin in diabetic neuropathy In cultured sensory neurons, NGF has been shown to increase neuritin mRNA and protein in a dose-dependent manner. NGF treatment reverses the decrease in neuritin levels in the DRGs of STZ-induced diabetic rats[10]. In addition, NGF levels are reduced in the diabetic PNS[23,24]. These results indicate that exogenous NGF may promote axonal regeneration in human diabetic patients via neuritin induction.
Androgen has been shown to induce axonal regeneration after the axotomy of various motor neurons[25]. After axotomy of the hamster facial nerve, exogenous androgen treatment lead to functional recovery by axonal regeneration[26]. Androgen treatment after axotomy of the facial nerve increased the expression of neuritin mRNA in vivo[11]. This androgen-dependent increase in neuritin mRNA was blocked by an antagonist of the androgen receptor[27]. Androgen-induced neuritin expression may participate in the axonal regeneration of other motor neuron injuries. Androgen may induce neuritin expression through a different mechanism than NGF signaling does. The neuritin promoter harbors seven putative androgen response elements[27], suggesting that the androgen receptor may directly control neuritin gene expression in motor neurons.
Neuritin mRNA has also been observed to increase after spinal cord injury. Fourteen days after injury by the weight-drop method, the amount of neuritin mRNA peaked near the injury site[28]. This finding may suggest that neuritin contributes to axonal regeneration in the CNS as well as in the PNS; however, future experiments are required to provide more definitive evidence. In addition, many questions remain regarding the molecular mechanisms by which neuritin expression is regulated under each pathological condition.
Neuritin was first identified as a neural-activity-regulated gene product in the brain. In addition to the induction following spinal cord injury, neuritin expression is also drastically increased in the hippocampus in models of epilepsy and brain ischemia (Figure 3).
In temporal lobe epilepsy, granule cells in the hippocampal dentate gyrus show axonal sprouting and branching. This axonal change, which is called mossy fiber sprouting, is one of the hallmarks of an epileptic hippocampus observed in both experimental animal models[29-33] and clinical cases[34-38]. Mossy fiber sprouting leads to axonal projections into the inner molecular layer of the dentate gyrus and results in the formation of a closed recurrent circuit[29,39-42]. The formation of a new excitatory circuit within the dentate granule cells induces spontaneous bursting in the dentate gyrus under certain conditions[33,43-45]. The idea that mossy fiber sprouting is the main reason for acquired epileptogenesis is controversial; however, it is probable that increased axonal branching in the granule cells results in the formation of epileptogenic recurrent circuits in the hippocampus[46,47]. We have observed that neuritin promotes the arborization and elongation of mossy fibers in hippocampal slice cultures (unpublished data). This neuritin-induced mossy fiber sprouting may contribute to the formation of closed circuits between the granule cells (Figure 3). In this instance, an upregulation of neuritin may be harmful to brain function. Inhibition of neuritin function could be necessary for the suppression of epileptogenesis in temporal lobe epilepsy.
Transient middle cerebral artery occlusion (MCAO) leads to permanent damage of the brain that affects the lateral striatum and the lateral parietal, temporal and occipital cortex[48]. Two hours after MCAO treatment in rats, an induction of neuritin mRNA was observed in the ipsilateral dentate gyrus of the hippocampus, as well as in the ipsilateral cerebral cortex[49]. The increase in the hippocampus was robust and confined to this area 6 h after reperfusion[49]. In this model, the significance of neuritin induction in the cortex remains unclear; however, the upregulation of neuritin in dentate granule cells might indicate its involvement in the pathogenesis of postischemic epilepsy through mossy fiber sprouting (Figure 3).
Thus, aberrant neuritogenesis, axonal branch formation, and axonal elongation by neuritin could exacerbate neuronal dysfunction in the CNS. Tightly controlled regulation of neuritin expression may be required for the treatment of various CNS diseases.
Neuritin is a small extracellular protein that is expressed in neurons, and its expression is upregulated by neuronal activity. The expression of neuritin increases in response to neuronal damage and neurotrophin treatment in the CNS and the PNS. Neuritin expression leads to morphological changes in neurons. The induction of neuritogenesis, the enhancement of neurite arborization, and the promotion of neurite extension are all elicited by neuritin. These morphological changes suggest that neuritin can contribute to axonal regeneration after axotomy. A variety of studies provide evidence that neuritin may contribute to axonal regeneration in PNS and CNS injuries. In contrast, neuritin induction in the hippocampus in response to epileptic seizures or brain ischemia may aggravate neuronal damage by enhancing mossy fiber sprouting. Thus, tightly controlled regulation of appropriate neuritin levels will be useful in the treatment of axonal pathology.
P- Reviewer: Osaka H S- Editor: Zhai HH L- Editor: A E- Editor: Liu XM
| 1. | David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931-933. [PubMed] |
| 2. | Ribotta MG, Provencher J, Feraboli-Lohnherr D, Rossignol S, Privat A, Orsal D. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J Neurosci. 2000;20:5144-5152. [PubMed] |
| 3. | Pearse DD, Marcillo AE, Oudega M, Lynch MP, Wood PM, Bunge MB. Transplantation of Schwann cells and olfactory ensheathing glia after spinal cord injury: does pretreatment with methylprednisolone and interleukin-10 enhance recovery. J Neurotrauma. 2004;21:1223-1239. [PubMed] |
| 4. | Nedivi E, Hevroni D, Naot D, Israeli D, Citri Y. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993;363:718-722. [PubMed] |
| 5. | Naeve GS, Ramakrishnan M, Kramer R, Hevroni D, Citri Y, Theill LE. Neuritin: a gene induced by neural activity and neurotrophins that promotes neuritogenesis. Proc Natl Acad Sci USA. 1997;94:2648-2653. [PubMed] |
| 6. | Nedivi E, Fieldust S, Theill LE, Hevron D. A set of genes expressed in response to light in the adult cerebral cortex and regulated during development. Proc Natl Acad Sci USA. 1996;93:2048-2053. [PubMed] |
| 7. | Harwell C, Burbach B, Svoboda K, Nedivi E. Regulation of cpg15 expression during single whisker experience in the barrel cortex of adult mice. J Neurobiol. 2005;65:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR, Duman RS. Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007;13:1476-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, Duman RS. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci. 2003;23:10841-10851. [PubMed] |
| 10. | Karamoysoyli E, Burnand RC, Tomlinson DR, Gardiner NJ. Neuritin mediates nerve growth factor-induced axonal regeneration and is deficient in experimental diabetic neuropathy. Diabetes. 2008;57:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Fargo KN, Alexander TD, Tanzer L, Poletti A, Jones KJ. Androgen regulates neuritin mRNA levels in an in vivo model of steroid-enhanced peripheral nerve regeneration. J Neurotrauma. 2008;25:561-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Putz U, Harwell C, Nedivi E. Soluble CPG15 expressed during early development rescues cortical progenitors from apoptosis. Nat Neurosci. 2005;8:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Sato H, Fukutani Y, Yamamoto Y, Tatara E, Takemoto M, Shimamura K, Yamamoto N. Thalamus-derived molecules promote survival and dendritic growth of developing cortical neurons. J Neurosci. 2012;32:15388-15402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Cappelletti G, Galbiati M, Ronchi C, Maggioni MG, Onesto E, Poletti A. Neuritin (cpg15) enhances the differentiating effect of NGF on neuronal PC12 cells. J Neurosci Res. 2007;85:2702-2713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Nedivi E, Wu GY, Cline HT. Promotion of dendritic growth by CPG15, an activity-induced signaling molecule. Science. 1998;281:1863-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 175] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Javaherian A, Cline HT. Coordinated motor neuron axon growth and neuromuscular synaptogenesis are promoted by CPG15 in vivo. Neuron. 2005;45:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Fujino T, Wu Z, Lin WC, Phillips MA, Nedivi E. cpg15 and cpg15-2 constitute a family of activity-regulated ligands expressed differentially in the nervous system to promote neurite growth and neuronal survival. J Comp Neurol. 2008;507:1831-1845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Yao JJ, Gao XF, Chow CW, Zhan XQ, Hu CL, Mei YA. Neuritin activates insulin receptor pathway to up-regulate Kv4.2-mediated transient outward K+ current in rat cerebellar granule neurons. J Biol Chem. 2012;287:41534-41545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Bisby MA. Axonal transport of labeled protein and regeneration rate in nerves of streptozocin-diabetic rats. Exp Neurol. 1980;69:74-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Longo FM, Powell HC, Lebeau J, Gerrero MR, Heckman H, Myers RR. Delayed nerve regeneration in streptozotocin diabetic rats. Muscle Nerve. 1986;9:385-393. [PubMed] |
| 21. | Sima AA, Bril V, Nathaniel V, McEwen TA, Brown MB, Lattimer SA, Greene DA. Regeneration and repair of myelinated fibers in sural-nerve biopsy specimens from patients with diabetic neuropathy treated with sorbinil. N Engl J Med. 1988;319:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 242] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Britland ST, Young RJ, Sharma AK, Clarke BF. Association of painful and painless diabetic polyneuropathy with different patterns of nerve fiber degeneration and regeneration. Diabetes. 1990;39:898-908. [PubMed] |
| 23. | Hellweg R, Hartung HD. Endogenous levels of nerve growth factor (NGF) are altered in experimental diabetes mellitus: a possible role for NGF in the pathogenesis of diabetic neuropathy. J Neurosci Res. 1990;26:258-267. [PubMed] |
| 24. | Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut RE, Sinicropi DV. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med. 1996;2:703-707. [PubMed] |
| 25. | Fargo KN, Foecking EM, Jones KJ, Sengelaub DR. Neuroprotective actions of androgens on motoneurons. Front Neuroendocrinol. 2009;30:130-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Jones KJ, Brown TJ, Damaser M. Neuroprotective effects of gonadal steroids on regenerating peripheral motoneurons. Brain Res Brain Res Rev. 2001;37:372-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Marron TU, Guerini V, Rusmini P, Sau D, Brevini TA, Martini L, Poletti A. Androgen-induced neurite outgrowth is mediated by neuritin in motor neurones. J Neurochem. 2005;92:10-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Di Giovanni S, Faden AI, Yakovlev A, Duke-Cohan JS, Finn T, Thouin M, Knoblach S, De Biase A, Bregman BS, Hoffman EP. Neuronal plasticity after spinal cord injury: identification of a gene cluster driving neurite outgrowth. FASEB J. 2005;19:153-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Wenzel HJ, Woolley CS, Robbins CA, Schwartzkroin PA. Kainic acid-induced mossy fiber sprouting and synapse formation in the dentate gyrus of rats. Hippocampus. 2000;10:244-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Kotti T, Riekkinen PJ, Miettinen R. Characterization of target cells for aberrant mossy fiber collaterals in the dentate gyrus of epileptic rat. Exp Neurol. 1997;146:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 32. | Okazaki MM, Evenson DA, Nadler JV. Hippocampal mossy fiber sprouting and synapse formation after status epilepticus in rats: visualization after retrograde transport of biocytin. J Comp Neurol. 1995;352:515-534. [PubMed] |
| 33. | Represa A, Jorquera I, Le Gal La Salle G, Ben-Ari Y. Epilepsy induced collateral sprouting of hippocampal mossy fibers: does it induce the development of ectopic synapses with granule cell dendrites. Hippocampus. 1993;3:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 156] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Kandratavicius L, Hallak JE, Young LT, Assirati JA, Carlotti CG, Leite JP. Differential aberrant sprouting in temporal lobe epilepsy with psychiatric co-morbidities. Psychiatry Res. 2012;195:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Lynd-Balta E, Pilcher WH, Joseph SA. AMPA receptor alterations precede mossy fiber sprouting in young children with temporal lobe epilepsy. Neuroscience. 2004;126:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | El Bahh B, Lespinet V, Lurton D, Coussemacq M, Le Gal La Salle G, Rougier A. Correlations between granule cell dispersion, mossy fiber sprouting, and hippocampal cell loss in temporal lobe epilepsy. Epilepsia. 1999;40:1393-1401. [PubMed] |
| 37. | Zhang N, Houser CR. Ultrastructural localization of dynorphin in the dentate gyrus in human temporal lobe epilepsy: a study of reorganized mossy fiber synapses. J Comp Neurol. 1999;405:472-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Mathern GW, Pretorius JK, Babb TL, Quinn B. Unilateral hippocampal mossy fiber sprouting and bilateral asymmetric neuron loss with episodic postictal psychosis. J Neurosurg. 1995;82:228-233. [PubMed] |
| 39. | Buckmaster PS, Zhang GF, Yamawaki R. Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J Neurosci. 2002;22:6650-6658. [PubMed] |
| 40. | Cavazos JE, Zhang P, Qazi R, Sutula TP. Ultrastructural features of sprouted mossy fiber synapses in kindled and kainic acid-treated rats. J Comp Neurol. 2003;458:272-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Isokawa M, Fried I. Extracellular slow negative transient in the dentate gyrus of human epileptic hippocampus in vitro. Neuroscience. 1996;72:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Patrylo PR, Schweitzer JS, Dudek FE. Abnormal responses to perforant path stimulation in the dentate gyrus of slices from rats with kainate-induced epilepsy and mossy fiber reorganization. Epilepsy Res. 1999;36:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321-330. [PubMed] |
| 44. | Wuarin JP, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rats. J Neurosci. 1996;16:4438-4448. [PubMed] |
| 45. | Patrylo PR, Dudek FE. Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats. J Neurophysiol. 1998;79:418-429. [PubMed] |
| 46. | Hunt RF, Scheff SW, Smith BN. Regionally localized recurrent excitation in the dentate gyrus of a cortical contusion model of posttraumatic epilepsy. J Neurophysiol. 2010;103:1490-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | Sutula TP, Dudek FE. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog Brain Res. 2007;163:541-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 48. | Memezawa H, Minamisawa H, Smith ML, Siesjö BK. Ischemic penumbra in a model of reversible middle cerebral artery occlusion in the rat. Exp Brain Res. 1992;89:67-78. [PubMed] |
| 49. | Rickhag M, Teilum M, Wieloch T. Rapid and long-term induction of effector immediate early genes (BDNF, Neuritin and Arc) in peri-infarct cortex and dentate gyrus after ischemic injury in rat brain. Brain Res. 2007;1151:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Tuszynski MH, Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron. 2012;74:777-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |